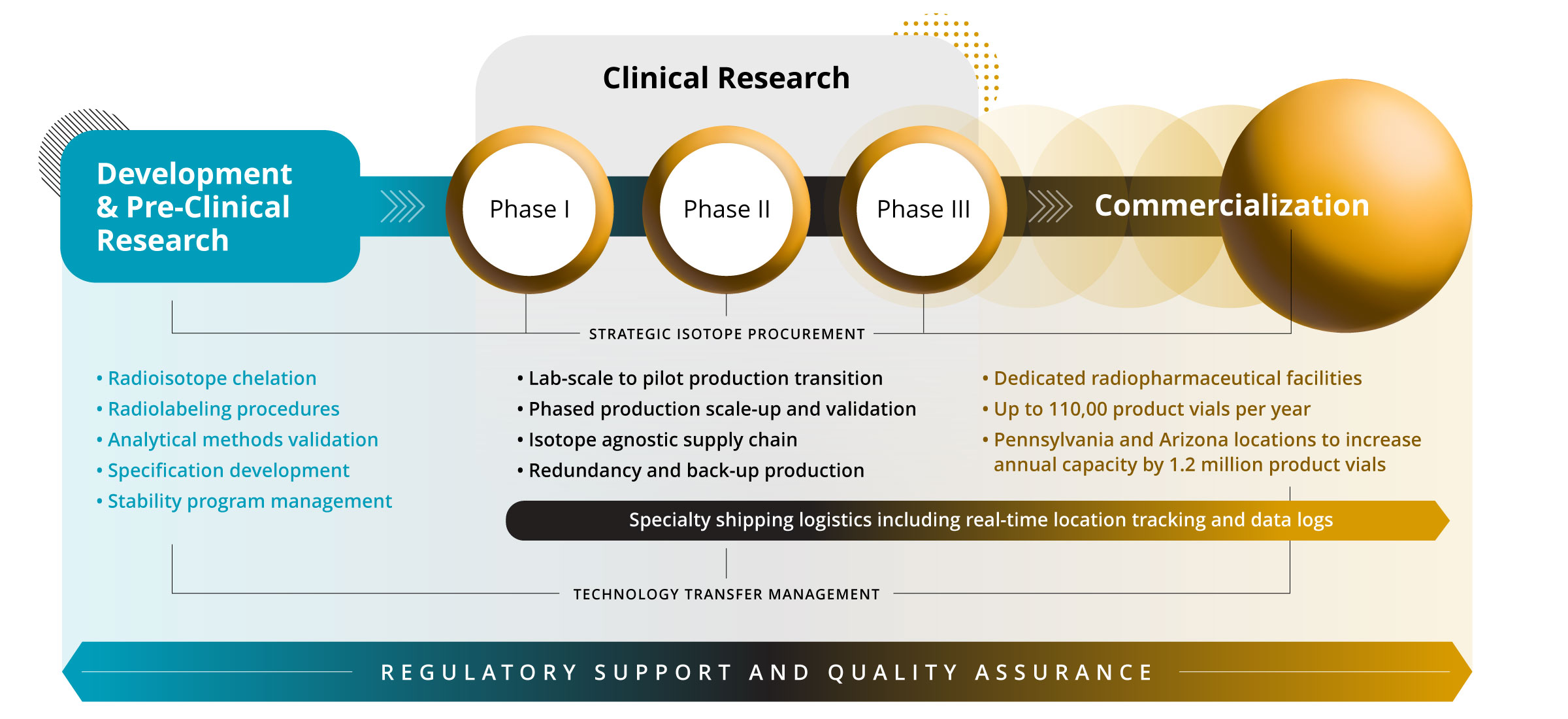
Every detail of our state-of-the-art, commercial-scale facilities is built with one purpose in mind: Advancing the development, manufacturing, and delivery of our clients’ radiotherapies quickly, efficiently, and safely.
Our Rochester, MN facility and upcoming Philadelphia, PA and Phoenix, AZ expansion sites uniquely equip us to mitigate current bottlenecks and bring your radiotherapeutics to market—and closer to patients—faster without sacrificing quality.
With our upcoming expansion sites, we’re scaling to over 100,000 square feet—purpose-built for radiopharmaceutical development and manufacturing—establishing one of the largest networks of its kind anywhere in the world.