Radiopharmaceutical therapies require a completely different approach to drug delivery—slow, highly controlled infusions, radiation shielding, and strict adherence to regulatory standards.
Yet most treatment centers are currently adapting tools that were never designed for this purpose. Manual syringe pushes, gravity drips, and retrofitted chemo pumps remain the norm, not because they’re ideal, but because nothing better has been available.
Nucleus RadioPharma is closing that gap with the first infusion system engineered specifically for the complexities of radiopharmaceutical therapy.
The Problem: New Medicine, Old Tools
Therapeutic radiopharmaceuticals must be handled with precision. Due to radioactive decay, doses are often patient-specific, volume-sensitive, and time-critical. They also emit radiation that requires shielding for both patients and staff.
In the absence of purpose-built equipment, clinicians are left with three main delivery methods:
- Syringe push: A technologist manually administers the dose from a syringe, allowing for some control. However, it significantly increases radiation exposure to the operator and relies on perfect manual consistency to avoid dosing errors.
- Gravity drip: The radiopharmaceutical flows from an elevated vial through IV tubing. It’s simple and inexpensive, but it lacks fine flow control, making infusion times and dose accuracy difficult to manage. It also requires constant attention from staff.
- Standard infusion pumps (often designed for chemotherapy): These offer more consistent flow but aren’t built for radioactive materials. Hospitals must resort to workaround setups, such as placing pumps behind shielding or outside the room using long IV lines.
Each of these methods is a compromise. They expose staff to radiation, require extra oversight, and often fail to meet the sterility and documentation standards required for regulatory approval and reimbursement. And in a healthcare environment facing staffing shortages and rising patient volumes, these inefficiencies become a real barrier to broader adoption.
The Solution: A System Designed for Radiopharma from the Ground Up
To eliminate those compromises, Nucleus RadioPharma has developed a new infusion system specifically for radiopharmaceutical therapies—one designed with input from nuclear medicine technologists and oncology nurses to ensure it fits seamlessly into clinical practice.
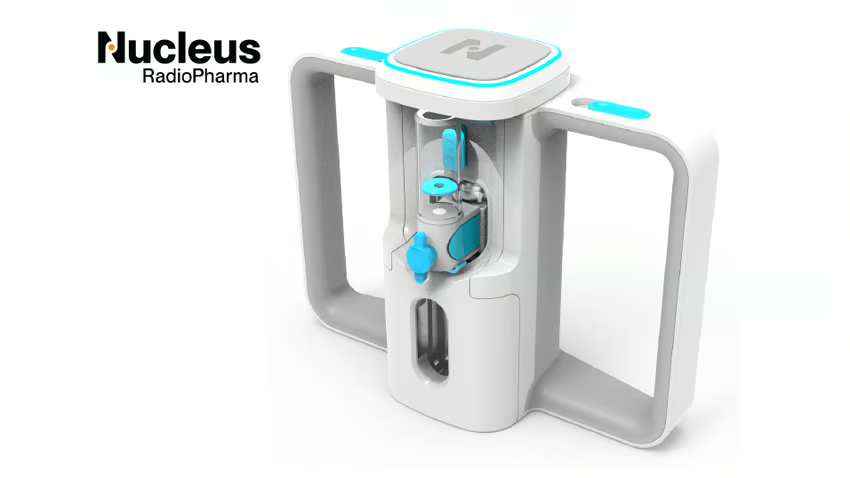
A key innovation of the system is its disposable, shielded fluid cartridge, which snaps directly into any standard IV infusion pump. Its passive design allows the pump to control flow and volume, requiring no additional hardware or special tools. This setup enables slow, precise infusions while significantly reducing radiation exposure for both patients and staff.
Every design choice (down to how the cartridge fits inside a dose calibrator well) was made to improve safety, accuracy, and efficiency at every step.
Key features include:
- Compatibility with all major radiation types (alpha, beta, gamma)
- Precise dosing with support for partial vial withdrawal
- Snap-on connection that reduces puncture risk and simplifies setup
- Built-in flush functionality to reduce waste and improve dose accuracy
- Post-infusion residual measurement using standard dose calibrators
- Mountable on standard IV poles
- Designed for easy disinfection with hospital-standard cleaners
- Sterile, Class II FDA medical device designation (pending)
Because the system works with existing IV pumps, tubing, and calibrators, it integrates easily into current workflows, reducing setup time, minimizing manual handling, and simplifying training. That ease of use is especially critical as staffing shortages and patient volumes continue to grow.
It also supports compliance in an increasingly complex regulatory landscape. With standards like USP<825> raising expectations for sterility, dose tracking, and radiation safety, manual methods often fall short. Our system enables direct-from-vial infusion, accurate residual measurement, and automated flush cycles—features that streamline documentation and support payer reimbursement.
It increases clinician confidence in dosing, supports hospital throughput, and helps ensure safer, more consistent patient access to advanced therapies.
More Than a CDMO
Radiopharmaceutical therapies bring unique challenges to the point of care. Our new infusion system is designed to meet those challenges head-on, making it easier for providers to deliver radiotherapies safely, efficiently, and at scale.
The system is currently in alpha development, on track for regulatory submission in 2025, and is expected to launch commercially in 2026.
As we continue to expand what’s possible in radiopharmaceutical delivery, we’re proving that Nucleus RadioPharma is more than a CDMO. We’re building the technologies and infrastructure needed to develop and deliver radiotherapies successfully.
Developing the next breakthrough therapy? Let’s talk about how we can support you from early development through clinical delivery.